Solutions
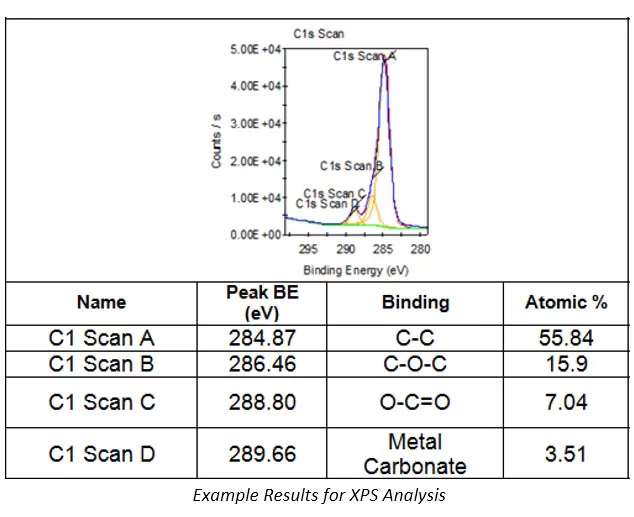
XPS Analysis
Electron Spectroscopy for Chemical Analysis (ESCA) or X-ray Photoelectron Spectroscopy (XPS) is a failure analysis technique primarily used in the identification of compounds on the surface of a sample.
It utilizes X-Rays with low energy (typically 1-2 keV) to knock off photoelectrons from atoms of the sample through the photoelectric effect. The energy content of these ejected electrons are then analyzed by a spectrometer to identify the elements where they came from.
The incident X-Rays used in knocking off the electrons must possess energy that is both monochromatic and of accurately known magnitude. The X-ray source material must also be a light element since X-ray line widths, which must be as narrow as possible in ESCA, are proportional to the atomic number of the source material. It is for these reasons that commercial XPS systems typically use the K-alpha X-rays of aluminum (Al K-alpha E = 1.487 keV) and magnesium (Mg K-alpha E = 1.254 keV).
Although the X-rays penetrate deep into the sample, only the electrons on the surface of the sample are able to escape without significant loss of energy for analysis. As such, ESCA, just like AES, is basically a surface analysis technique.